How do controlled release drug delivery systems work?
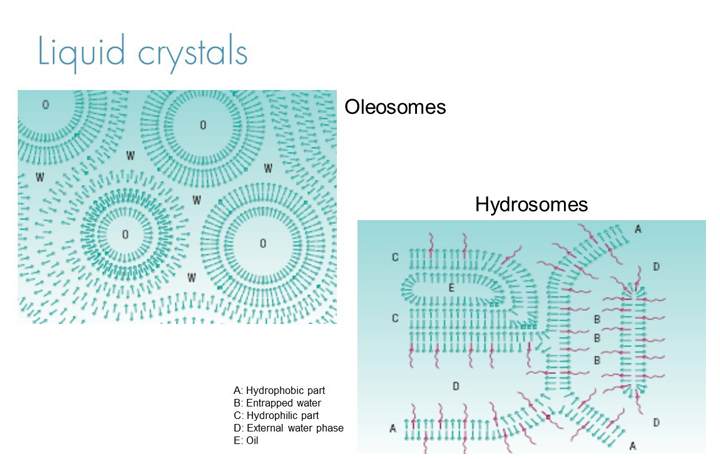
Topical formulations made with the appropriate emulsifiers can form liquid crystals with an organised lamellar structure around the oil droplets of oil in water emulsions and the aqueous continuous phase. This structure creates “pockets” of API which is then gradually released as the formulation dries down on the skin surface after application.
In addition, the liquid crystal systems can help improve skin hydration which in turn means API’s become more permeable through the stratum corneum, increasing the percentage of the applied dose to make it through the stratum corneum to take effect in the lower levels of the epidermis or dermis. There are two types of liquid crystal emulsions, oleosome which tend to offer excellent controlled release of the active over long periods while still delivering close to the full dose and hydrosomes which offer increased hydration making them particularly suited to hydrophilic actives.
In addition, liquid crystal emulsions offer superior stability in topical oil in water formulations with large steric barriers to coalescence. Therefore, these systems usually offer the most stable oil water emulsions possible making them excellent for formulation with formulation destabilising API’s, high active levels and formulations with high levels of polar solvents such as propylene glycol, ethanol, glycerine and dimethyl isosorbide.
For controlled release in in oral dosage forms glycerol mono-oleate (GMO) is an effective controlled release drug delivery system, which forms a stable viscous gel matrix in water/ gastric fluid that releases a dissolved or dispersed drug by slow diffusion. At higher water levels, lipid and aqueous domains coexist in the matrix allowing hydrophilic, lipophilic, and amphiphilic drugs to be incorporated. When administered orally in the form of a gelatin capsule, GMO encounters the gastrointestinal fluids following dissolution of the capsule and transforms into the viscous phase, promoting sustained release of the active.
Liquid Crystal Emulsification Systems for topical applications
Liquid crystal emulsification systems are useful as controlled release agents in topical applications. The controlled release of the active prolongs skin hydration and enables patients to apply the product less often. This reduces the frequency of handling compromised skin and the likelihood of missed applications, as well as improving convenience.
Liquid crystal emulsification systems also result in a more stable formulation and an aesthetically pleasing skin feel.
Liquid crystals
Effects on delivery - lipophilic actives

Effect on delivery - hydrophilic actives

Our products recommended for controlled release:
Brij™ S721 Pharma
 Featured
Featured
Crodafos™ CES pharma

Crodamol™ CAP
.jpg&mn=healthcare&w=768&xr=0&yr=0&xfp=6&yfp=6&hash=B7A014F4ED7B52AFF22F83F2BEDE8A5919C138999FEC74AA)
