Non-animal derived, Sustainable Squalene in GMP quality
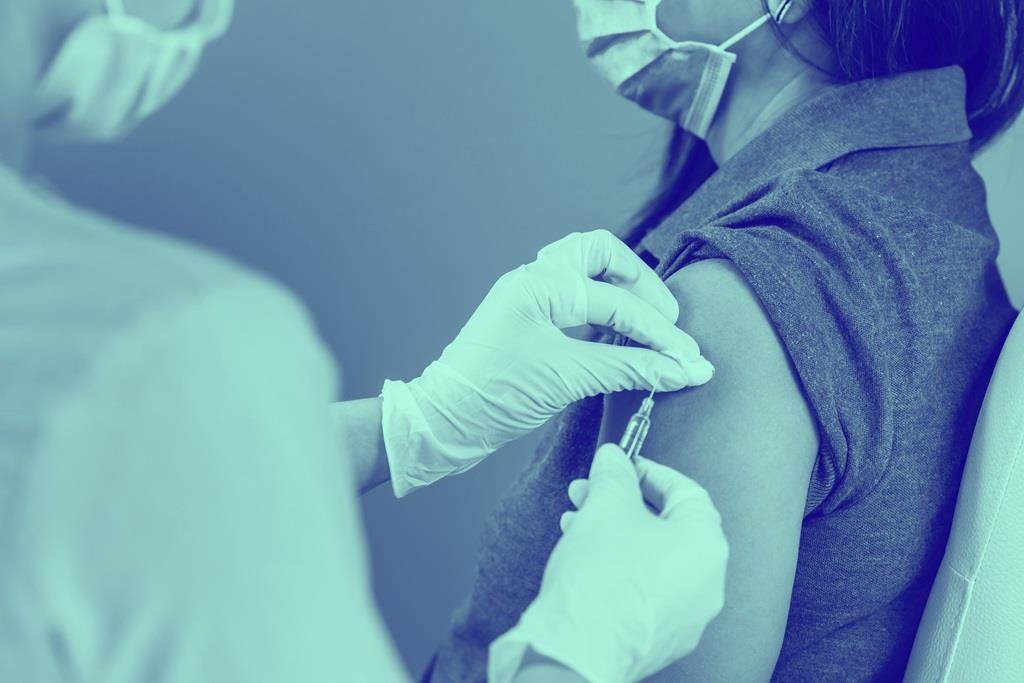
Squalene is a critical ingredient in emulsion adjuvants used for seasonal and pandemic influenza vaccines. Its growing importance is underscored by its role in clinical development projects for prophylactic vaccines targeting tuberculosis, shingles, schistosomiasis, HIV, and other severe diseases—highlighting a strong and increasing demand for high-quality squalene.
Ecological concerns with traditional squalene sources
To date, the stringent purity requirements of the vaccine industry have largely been met by squalene sourced from liver oil extracted from deep-sea sharks. However, this approach poses significant ecological risks, threatening shark populations that are vital to marine ecosystems and global blue carbon balance.
A sustainable, superior alternative to animal derived squalene
Our Sustainable Squalene in GMP Quality leverages cutting-edge synthetic biology and fermentation technology to provide an innovative, environmentally friendly alternative. This advanced process yields pharmaceutical-grade squalene of highest purity, tailored for vaccine formulations.
- Superior Purity: The final purification step ensures that all test parameters and specification limits required by the European Pharmacopoeia (Ph. Eur.) monograph 2805 are met. With a purity of at least 99%, our Squalene meets the demanding specifications required by the vaccine industry.
Squalene aligned with sustainability and global priorities
The shift toward sustainable solutions aligns with global priorities for environmental conservation and stable supply chains, particularly in the production of lifesaving vaccines.
Key benefits include:
- Environmental Preservation: By eliminating reliance on shark-derived sources, our Sustainable Squalene helps conserve marine ecosystems and protect blue carbon reservoirs.
- Low Environmental Impact: The raw materials used in our production process have minimal land-use impact. A comprehensive Product Carbon Footprint study has been conducted to evaluate its climate impact.
- Supply Chain Stability: Fermentation-based production ensures a consistent, reliable, scalable, and ethical supply chain, critical for responding to seasonal and pandemic vaccine demands.
Sustainable Squalene available for commercial vaccine production
Our Sustainable Squalene, produced to GMP standards, is ready for use in the commercial manufacturing of vaccines - offering superior purity, ecological benefits, and a sustainable future for vaccine development.
Find more information like this in our Frequently Asked Questions here.
ORDER YOUR SAMPLE TODAY!
.jpg&mn=healthcare&w=768&xr=0&yr=0&xfp=6&yfp=6&hash=759CB83702D0BA63DE2DB96F3DEFB82519C138999FEC74AA) Featured
Featured
Sustainably Sourced Squalene
Um histórico de apoio às vacinas

Comprometidos com o sucesso da sua vacina
Frequently asked questions
Squalene is a natural lipid belonging to the terpenoid family. Its name is derived from the Latin name squalus for shark.
Most plants, fungi, and animals produce squalene as a biochemical precursor in sterol biosynthesis, including cholesterol and steroid hormones in the human body.
Its major role currently is in influenza vaccines. It is utilised in squalene-based emulsion adjuvants in several seasonal vaccines such as FLUAD™ and FLUAD™ Tetra as well as pandemic preparedness vaccines such as Pandemrix™, Prepandrix™ and Humenza™.
Clinical development with vaccine formulations using a combination of PHAD™ and squalene as adjuvant system is ongoing. AAHI (The Access to Advanced Health Institute) has developed their GLA-SE* adjuvant formulation currently included in various vaccine development projects such as tuberculosis, malaria, HIV, schistosomiasis, and herpes zoster.
Currently, all marketed vaccines use shark-derived squalene.
*GLA = Glucopyranosyl Lipid A (equal to PHAD)
It is incorporated in squalene-based emulsions, together with surfactants and other lipids. Tween™ 80, Span™ 85, Synperonic PE/F 68 (Poloxamer 188) or derivatives of phosphatidylcholine, which are available from Avanti (for example DSPC, DMPC, DLPC, DOPC). It can also be combined with PHAD™.
Contact our experts if you would like to discuss .
In short, the benefits of squalene-based emulsions are as follows:
- Established safety profile.
- High tolerance in both adults and children.
- Increased vaccine immunogenicity.
- Dose sparing effects.
- Broadened cross-strain neutralisation in influenza vaccines.
a. Addition of vitamin E as an antioxidant.
b. Filling the product under a nitrogen blanket.
We have two variants available, squalene with and without vitamin E. Both variants will be under nitrogen blanket. The content of vitamin E, serving as an antioxidant, is between 400 and 600ppm. This amount of vitamin E is not sufficient for immunostimulatory activity.
The reliance of animal derived squalene could risk being banned in the future. Non-animal derived squalene offers not only a more sustainable but also a more robust supply chain since it eliminates the supply risk of a shark fishing ban or shark extinction.
2. High purity
Our sustainably sourced Squalene distinguishes itself in terms of purity from other non-shark-derived squalene products available, to our knowledge. Our Squalene not only satisfies the Ph.Eur. monograph requirements with its ≥99% purity but is comparable to shark-derived squalene which equally has a purity >99%, making it well-suited for use in vaccines.
3. Consistency
As a well-controlled fermentation process is used to obtain squalene, there is high consistency, this is low batch-to-batch variability.
4. Sustainability
Our partner Amyris' expertise in synthetic biology in conjunction with our purification excellence gives access to a sustainable source that contributes to an environmentally-friendly production without endangering shark populations which play a key role in marine ecosystems.

Vaccines for a more sustainable future
EXCLUSIVE WHITEPAPER
The vaccines of tomorrow revolve around sustainable supply chains and access to scalable commercial supplies that do not put undue pressure on the natural environment. It is our responsibility to future-proof vaccines by focusing on longevity in our processes. With combined global efforts, we can enhance the global health infrastructure, leading us to develop better vaccines against more diseases and supporting our preparedness for future outbreaks.
We believe that lobal collaborations are the cornerstone of creating a greener planet. Let's do all we can today, to generate a sustainable future we're proud of.
Get in touch to discuss your sample

Adjuvant Systems
Sustainable saponins (2024)
Sustainable vaccines
2.https://www.tandfonline.com/doi/full/10.1080/21645515.2017.1415684
3. https://doi.org/10.1038/s41586-020-03173-9
4. https://doi.org/10.1038/s41586-020-03173-9
