Proprietary purification process extends formulation shelf life
Formulating generic drugs of equivalent stability, safety, bioequivalence, and dosage form as originator drugs can be a great challenge. James Humphrey, our Research and Technology Specialist explores how excipients are used to better formulate generic drugs and how our proprietary purification process can extend formulation shelf life.
Generic drugs must be formulated to have equivalent stability, safety, bioequivalence, route of administration, and dosage form as their originators. While the active ingredient of generic drugs remains the same, formulating with the correct excipients to meet these standards can be a challenge, due to poor solubility, physical or chemical instability and ineffective drug delivery, says James Humphrey.
“Excipients can provide additional benefits in the manufacture of generic drugs above and beyond the necessities of stability and bioequivalence, such as reduced taste impact in pediatric oral medicines that can result in better patient compliance or reduced cross linking in soft gel capsules improving dissolution rates,” he says.
Generic drugs must be formulated to have equivalent stability, safety, bioequivalence, route of administration, and dosage form as their originators. While the active ingredient of generic drugs remains the same, formulating with the correct excipients to meet these standards can be a challenge, due to poor solubility, physical or chemical instability and ineffective drug delivery, says James Humphrey.
“Excipients can provide additional benefits in the manufacture of generic drugs above and beyond the necessities of stability and bioequivalence, such as reduced taste impact in pediatric oral medicines that can result in better patient compliance or reduced cross linking in soft gel capsules improving dissolution rates,” he says.
 Featured
Featured
Super Refined™ Polysorbate 80
Super Refined Polysorbates solubilizam e estabilizam os mais sensíveis ingredientes ativos em diversas formas de dosagens, incluindo oral e injetável.
O processo Super Refining remove impurezas...
 Featured
Featured
Super Refined™ PEG 300
Super Refined PEGs são solventes para uso com os mais sensíveis ingredientes ativos em diversas formas de dosagens, incluindo oral e injetável.
O processo Super Refining remove impurezas polares...
He adds that the removal of impurities in our Super Refined™ product offerings can deliver improved drug and formulation stability, a lower taste impact, and improved patient compliance. When formulating, oxidative stability is important to prevent the formation of peroxides and aldehydes, which are common oxidation products for a number of excipients. The presence of peroxides and aldehydes can leave APIs susceptible to degradation, impacting drug performance, stability, and shelf life. “Our Super Refined excipients, created by Croda’s proprietary purification process, extend the shelf life of pharmaceutical formulations through the removal of impurities that contribute to oxidative degradation.”
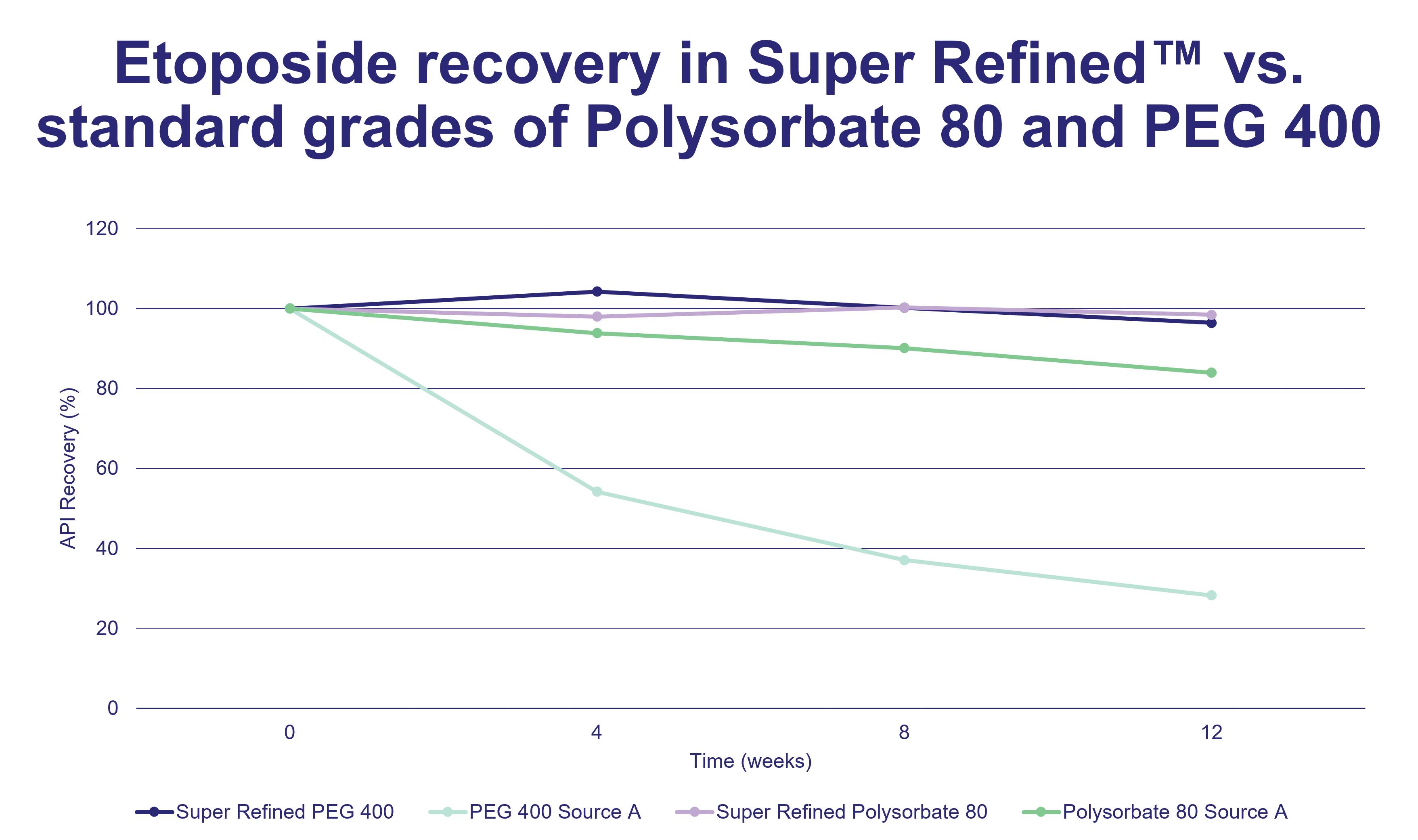
Figure 1: A graph to compare % etoposide recovery during an accelerated 12-week stability test at 40OC in Super Refined grades of PEG 400 and Polysorbate 80 compared to standard grades.
Etoposide is a chemotherapy drug used in the treatment of various cancers, including testicular, prostate, bladder, stomach, and lung cancer. Existing in two dosage forms – a concentrate for intravenous (IV) infusion and a soft-gel capsule for oral delivery – etoposide is a topoisomerase inhibitor. IV formulations of etoposide contain PEG 300 and Polysorbate 80.
“Our formulations team reformulated etoposide with Super Refined PEG 300 and Super Refined Polysorbate 80,” says James. An accelerated 12-week stability test at 40°C was performed to compare its stability in the Super Refined formulation versus standard excipients. Stability was measured in API recovery (%); results are shown in Figure 1.
“Etoposide was notably more stable in the Super Refined grades, demonstrating almost 100% recovery in Super Refined Polysorbate 80, and over 90% recovery in Super Refined PEG 300 after 12 weeks,” he says.
“Our formulations team reformulated etoposide with Super Refined PEG 300 and Super Refined Polysorbate 80,” says James. An accelerated 12-week stability test at 40°C was performed to compare its stability in the Super Refined formulation versus standard excipients. Stability was measured in API recovery (%); results are shown in Figure 1.
“Etoposide was notably more stable in the Super Refined grades, demonstrating almost 100% recovery in Super Refined Polysorbate 80, and over 90% recovery in Super Refined PEG 300 after 12 weeks,” he says.
“The increase in stability of etoposide in the Super Refined grade was particularly notable in PEG 300 where the standard grade had only 25% recovery after 12 weeks at 40°C. In summary, the use of Super Refined™ excipients when formulating can have a number of benefits including increased API stability, a shorter time to market, and improved patient compliance.”